Article contents
Immunological underpinnings of Ascaris infection, reinfection and co-infection and their associated co-morbidities
Published online by Cambridge University Press: 12 April 2021
Abstract
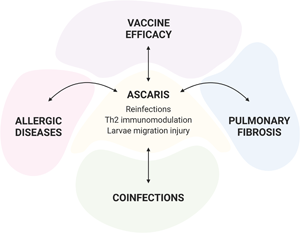
Human ascariasis is the most common and prevalent neglected tropical disease and is estimated that ~819 million people are infected around the globe, accounting for 0.861 million years of disability-adjusted life years in 2017. Even with the existence of highly effective drugs, the constant presence of infective parasite eggs in the environment contribute to a high reinfection rate after treatment. Due to its high prevalence and broad geographic distribution Ascaris infection is associated with a variety of co-morbidities and co-infections. Here, we provide data from both experimental models and humans studies that illustrate how complex is the interaction of Ascaris with the host immune system, especially, in the context of reinfections, co-infections and associated co-morbidities.
- Type
- Review Article
- Information
- Parasitology , Volume 148 , Special Issue 14: Lessons from studying roundworm and whipworm in the mouse - common themes and unique features , December 2021 , pp. 1764 - 1773
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
References
- 2
- Cited by





